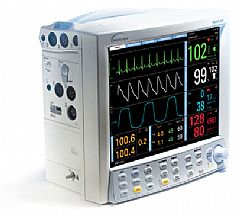
Datascope Product Detail
Model: Spectrum
Features:
- Spectrum’s 12.1 inch bright color display, with auto adjustable large numerics, makes viewing critical data simple
- Standard features include 8 auto-configurable waveforms, 3 or 5-lead ECG with ESU noise rejection, lead-selectable respiration, NIBP, Masimo SET motion tolerant SpO2, temperature, list, graph and OXY-CRG trends, and drug calculations
- Many options available including Nellcor SpO2, Microstream ETCO2, up to 4 invasive pressures, arrhythmia and ST analysis, cardiac output with hemodynamic calculations, and dual-trace recorder
- Using the External Parameter Module, add the extra parameters you need, including cardiac output, additional invasive blood pressures and temperature, without sacrificing portability
- Optional continuous Cardiac Output/SvO2 with interface to Edwards Vigilance Monitor
- Using the View 12 Analysis Module, print diagnostic quality 12-lead ECG data to a laser printer
- List and graphic trends store up to 120 patient time measurements, and are expandable to 500 entries with optional memory card
- Our exclusive Base Station, with its one-touch lever, allows you to connect/disconnect from various peripheral devices instantly
- Network to Panorama Central Station for continuous data storage
Specifications:
- Display
- Size: 12.1-inch (30.7cm) Color Active Matrix TFT LCD
- Resolution: 800 x 600 pixels
- Waveforms: 3 to 8
- ECG (3-Lead and 5 Lead)
- Leads: I, II, III, aVR, aVL, aVF, V
- Cable Detection: Autodetecting Datascope 3 or 5 wire
- Display Sensitivity: 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, cm/mV ± 10%
- Frequency Response To Screen
- Extended Mode: 0.05 – 100 Hz, -3db
- Monitor Mode: 0.5 – 40 Hz, -3db
- ST Mode: 0.05 – 40 Hz, -3db
- ECG Sync Pulse for Cardioversion:
- Delay: ≤35 ms max between QRS Peak and rising edge of Sync Pulse
- Amplitude: 2Vp minimum into a 5k ohm load
- Width: 2-7 ms
- Analog Output (ECG): Supports slaving of an IABP with ECG
- Delay: 25 ms max
- Sensitivity: 1 V/mV of input, ± 10%
- Defibrillator Overload Protection: Withstand 360 Joule discharge as per IEC 60601-2-27, 51.101.1
- Recovery Time: Time for recovery to within 1mV in < 8 seconds automatically. 3 seconds from 1Vpp at 60 Hz
-
ECG (12-Lead)
- Leads: I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
- Connection Type: Type II PCMCIA card to be installed in PCM slot
- Cable Detection: Automatically enabled when 12-lead Card is inserted
- Frequency Response
Extended Mode: 0.05 – 100 Hz, -3db @ 1mVpp input
- Monitor Mode: 0.5 – 40 Hz, -3db @ 1mVpp input (ESU filter disabled)
- ST Mode: 0.05 – 40 Hz, -3db @ 1mVpp input
- Analog Output (ECG): Disabled when 12-lead ECG analysis is enabled
- Defibrillator Overload Protection: Withstand 360 joule discharge as per IEC 60601-2-27, 51.101.1
-
Heart Rate Meter
- Range: 30 – 300 BPM Adult/Pediatric, 30 – 350 BPM Neonate 3-lead and 5-lead / 30 – 300 BPM 12-lead
- Accuracy: ± 3 BPM or ± 3% at 30 – 250 BPM whichever is greater, ± 5% at 251 – 350 BPM
- Pacer Rejection: Rejects all pulses of amplitude ± 2.0 mV to ± 700 mV and duration 0.1 to 2 ms with no tail. AAMI EC-13-1992 4.1.4 (3-Lead and 5-Lead) Rejects all pulses of amplitude ± 2.0 mV to 700 mV and duration 0.1 to 2 ms with 100 ms time constant tail of less than 2.0 mV, or 4 ms time constant tail of < 2.0 mV per AAMI EC-13-1992 3.1.4.2Tall T-Wave Rejection: Rejects all T-Waves of amplitude less than 120% of 1 mV, 100 ms QRS, T wave duration of 180 ms and Q-T interval of 350 ms per AAMI EC-13-1992 4.1.2.1 (c)
-
ST Analysis
- 3-lead or 5-lead Range: -9.9 mm to +9.9 mm
- 12-lead Absolute Range: -10 mm to +10 mm
- 12-lead Delta Range: -20 mm to +20 mm
- Resolution: 0.1 mm
- Default ST Measuring Point: 80 ms after J point for HR ≤ 120 BPM, 60 ms after J point for HR >120 BPM
-
Arrhythmia Analysis
- 3-lead or 5-lead cables: Adult / Pediatric Only: Asystole, Irregular Heart Rate, Couplets, Bigeminy, Trigeminy, Ventricular Tachycardia, Ventricular Fibrillation, PVC’s per minute, Runs, Ventricular Rhythm
- 12-lead: Adult / Pediatric Only: Asystole, Couplets, Runs, Bigeminy, Trigeminy, Ventricular Tachycardia, Ventricular Fibrillation, PVC’s per minute, Ventricular Rhythm, Pause
-
Respiration (ECG)
- Range: 4 to 199 BPM
- Accuracy: ± 2% or 2 breaths per minute whichever is greater from 4 to 150 BPM, ± 4% from 151 to 199 BPM
- Lead: I or II
-
Non-Invasive BP
- Technique: Oscillometric
- Systolic Range: Adult – 55-235 mmHg Pediatric – 55-160 mmHg Neonate – 45-120 mmHg
- Diastolic Range: Adult – 30-200 mmHg Pediatric – 30-150 mmHg Neonate – 20-100 mmHg
- Systolic Accuracy: Mean Error less than ± 5 mmHg, Standard Deviation less than ± 8 mmHg
- Diastolic Accuracy: Mean Error less than ± 5 mmHg, Standard Deviation less than ± 8 mmHg
- Pulse Rate Range: Adult/Pediatric: 35 to 245 BPM Neonate: 70 to 245 BPM
- Pulse Rate Accuracy: ± 3 BPM or 3% whichever is greater
- Connector Type: Rectus
- Cuff Inflation: Volume of 500 cc to 300 mmHg in ≤35 sec
-
Temperature
- Scale:Selectable C or F
- Range: 15 to 45C / 59 to 113F (T1 and T2) 0C to 5.5C / 0F to 9.9F (Delta T)
- Accuracy: ± 0.1C (15C to 45C) exclusive of probe errors. ± 0.2F (59F to 113F) exclusive of probe errors.
-
Pulse Oximetry
- Masimo SET SpO2 Accuracy Saturation with no motion conditions
- Adult/Pediatric: 70% to 100% ± 2 digits SpO2, 0-69% unspecified
- Neonate: 70% to 100% ± 3 digits SpO2, 0-69% unspecified
- Ear Sensor (Adult/Pediatric): 70% to 100% ± 4 digits SpO2, 0-69% unspecified
- Masimo SET SpO2 Accuracy Saturation during motion conditions
- Adult/Pediatric/Neonate: 70% to 100% ± 3 digits SpO2
- Response Time: 18 seconds to 95% of final step of % SpO2 value from 60-95% at 75 BPM. Averaging set at 8 seconds.
- Pulse Rate Range Masimo with no motion conditions
- Adult/Pediatric/Neonate: 30-235 ± 3 BPM, Ear Sensor (Adult/Pediatric) 30-235 ± 3 BPM
- Pulse Rate Range Masimo during motion conditions
- Adult/Pediatric/Neonate: 30-235 ± 5 BPM
- Low Perfusion Performance
- Masimo: > 0.02% Pulse Amplitude and % Transmission > 5% / Saturation (%SpO2) ± 2 digits; Pulse ± 3 digits
- Nellcor OxiMax SpO2 Saturation Accuracy
- Adult/Pediatric/Neonate:70% to 100% ± 3 digits
- Pulse Rate Range Nellcor: 20-249 ± 3 BPM
-
IBP
- Range: Sys/Dia/Mean -30 - +300 mmHg
- Accuracy: ± 2 mmHg or 2% whichever is greater
- Scale: -10 to 10, 0-20, 0-40, 0-160, 0-225, 0-320, 60-140 mmHg
- Zero Range: ± 120 mmHg
- Excitation: 5V DC ± 2%
- Frequency Response: DC to 16 Hz ± 1Hz, -3db
- Analog Output (IBP): Supports slaving of an IABP with IBP
- Delay: 25 ms max
- Sensitivity: 1 V/100 mmHg ± 10%
-
Recorder
- Speed: 3.125, 6.25, 12.5, 25 mm, and 50 mm/sec Note: 3.125 speed is for CO2 / Resp only.
-
CO2 (Microstream® Minimedi)
- Range: 0 – 99 mmHg
- Accuracy: 0 – 38 mmHg: ± 2 mmHg. 39 – 99 mmHg: ± 5% of reading + 0.8% for every 1 mmHg above 38 mmHg
- Respiration Rate: 0 – 150 breaths per minute
- Respiration Accuracy: 0 – 70 BPM: ± 1 BPM, 71 – 120 BPM: ± 2 BPM, 121 – 150 BPM: ± 3 BPM
- Sampling Rate: 50 ml/min ± 7.5 ml/min
-
Electrical Ratings
- AC Voltage: 100 – 240 VAC ± 10%, 50/60 Hz ± 3 Hz
- Battery Type: Lithium-ion
- Number of Batteries: 2
- Battery Voltage: 11.1 Vdc
- Battery Capacity: 4.4 Ah each
- Battery Run Time: 4 hrs 30 mins from two fully charged batteries at 25° C with ECG, SpO2 and 1 NIBP every 15 minutes, no recording, no CO2.
- Recharge Time: 5 hrs max
-
Environmental Conditions
- Storage Temperature: -20C - + 60C/-4F - +140F
- Storage Humidity: 10 – 95% non-condensing
- Operating Altitude: -1250 to 9889 feet ASL, 1060hPa to 700hPa, 795 mmHg to 525 mmHg
- Operating Temperature: 5C to 40C/41F - 104F
- Operating Humidity: 15% to 95%, non-condensing
-
Physical Dimensions:
- Monitor Size: 26.7cm H x 30.2cm W x 18.8cm D 10.5" H x 11.9" W x 7.4" D without module. 27.2cm H x 30.2cm W x 18.8cm D 10.7" H x 11.9" W x 7.5" D with module
- Weight: 5.43 kg (11.95 lbs) without optional accessories. 6.1 kg (13.52 lbs) with (2) lithium-ion batteries, without optional accessories. 0.8 kg (1.75 lbs) module, without optional accessories
-
Cardiac Output
- Range: 0.2 to 20.0 liters/min
- C.O. Repeatability: ± 2% or 0.02 l/min from the mean value
- Blood Temp Range: 17.5°C to 43°C (63.5°F to 109.4°F)
- Blood Temp Accuracy: ± 0.2°C (± 0.4°F) exclusive of probe errors
- Injectate Temp Range: -1.0°C to 30.0°C (30.2°F to 86°F )
- Injectate Temp Accuracy: ± 0.2°C (± 0.4°F) exclusive of probe errors
- Vigilance Interface: Supports the Vigilance IFM out communication protocol in accordance with Edwards Lifesciences specification ELS 1291 RevF
- Agency Compliances:
- Safety: IEC 60601-1:1988 + A1:1991 + A2:1995, UL 60601-1:2003, CSA Standard C22.2 No. 601.1M90, IEC 60601-1-1:2000/EN 60601-1-1:2001, IEC 60601-1-2:Ed. 2.1, IEC 60601-1-4:1996 + A1:1999/EN60601-1-4:1996 + A1:1999, EN 60601-2-30:2000/IEC 601-2-30:1999, EN 60601-2-34:2000/IEC 60601-2-34:2000, EN 60601-2-49:2001/IEC 60601-2-49:2001
- 3/5 Lead ECG: EN60601-2-25:1993/IEC 60601-2-25:1993 + A1:1999, section 36.202.101, EN 60601-2-27:1994/IEC 60601-2-27:1994
- 12 Lead ECG: EN 60601-2-25:1993/IEC 60601-2-25:1993 + A1:1999
- Hazard: EN ISO 14971:2000 + A1:2003
- Performance: EN 864:1996, EN 865:1997, EN 1060-1:1995 + A1:2002, EN 1060-3:1997 + A1:2005, AAMI EC13:1992, AAMI EC11:1991, AAMI EC53:1995, ISO 3744:1994, ANSI/AAMI/ISO 10993-10:1995, ANSI/AAMI/ISO 10993-1:1995, AAMI SP10:1996, EN475:1995
- Environmental: IEC 60068-2-6:1995 + Corrigendum 1, IEC 60068-2-27:1987, IEC 60068-2-64:1994 + Corrigendum 1, EN 60529:1991 + A1:2001 + Corrigendum 1, EN 55011:1998 + A1:1999 + A2:2002 (CISPR 11), EN 61000-3-2:2000 + A1:2001 + A2:2005, EN 61000-3-3:1995 + A1:2001 + A2:2005, EN 61000-4-6: 2003 + A1:2004 + A2:2006, EN 61000-4-3:2006, EN 61000-4-8:1993 + A1:2001, EN 61000-4-2:1995 + A1:1998 + A2:2001, EN 61000-4-4:2004, EN 61000-4-5:2005, EN 61000-4-11:2004, ECRI PB-296892:1979 (for Drop and Impact requirements),
- ISTA: 1994 procedure 1A, 2002/96/EC: 2003
Request Info
|